Summary: Researchers have successfully stabilized an enzyme that is able to degrade scar tissue as a result of spinal cord injury with the help of AI and robotics.
Source: Rutgers
By employing artificial intelligence (AI) and robotics to formulate therapeutic proteins, a team led by Rutgers researchers has successfully stabilized an enzyme able to degrade scar tissue resulting from spinal cord injuries and promote tissue regeneration.
The study, recently published in Advanced Healthcare Materials, details the team’s ground-breaking stabilization of the enzyme Chondroitinase ABC, (ChABC) offering new hope for patients coping with spinal cord injuries.
“This study represents one of the first times artificial intelligence and robotics have been used to formulate highly sensitive therapeutic proteins and extend their activity by such a large amount. It’s a major scientific achievement,” said Adam Gormley, the project’s principal investigator and an assistant professor of biomedical engineering at Rutgers School of Engineering (SOE) at Rutgers University-New Brunswick.
Gormley expressed that his research is also motivated, in part, by a personal connection to spinal cord injury.
“I’ll never forget being at the hospital and learning a close college friend would likely never walk again after being paralyzed from the waist down after a mountain biking accident,” Gormley recalled. “The therapy we are developing may someday help people such as my friend lessen the scar on their spinal cords and regain function. This is a great reason to wake up in the morning and fight to further the science and potential therapy.”
Shashank Kosuri, a biomedical engineering doctoral student at Rutgers SOE and a lead author of the study noted that spinal cord injuries, or SCIs, can negatively impact the physical, psychological, and socio-economic well-being of patients and their families. Soon after an SCI, a secondary cascade of inflammation produces a dense scar tissue that can inhibit or prevent nervous tissue regeneration.
The enzyme successfully stabilized in the study, ChABC, is known to degrade scar tissue molecules and promote tissue regeneration, yet it is highly unstable at the human body temperature of 98.6° F. and loses all activity within a few hours. Kosuri noted that this necessitates multiple, expensive infusions at very high doses to maintain therapeutic efficacy.
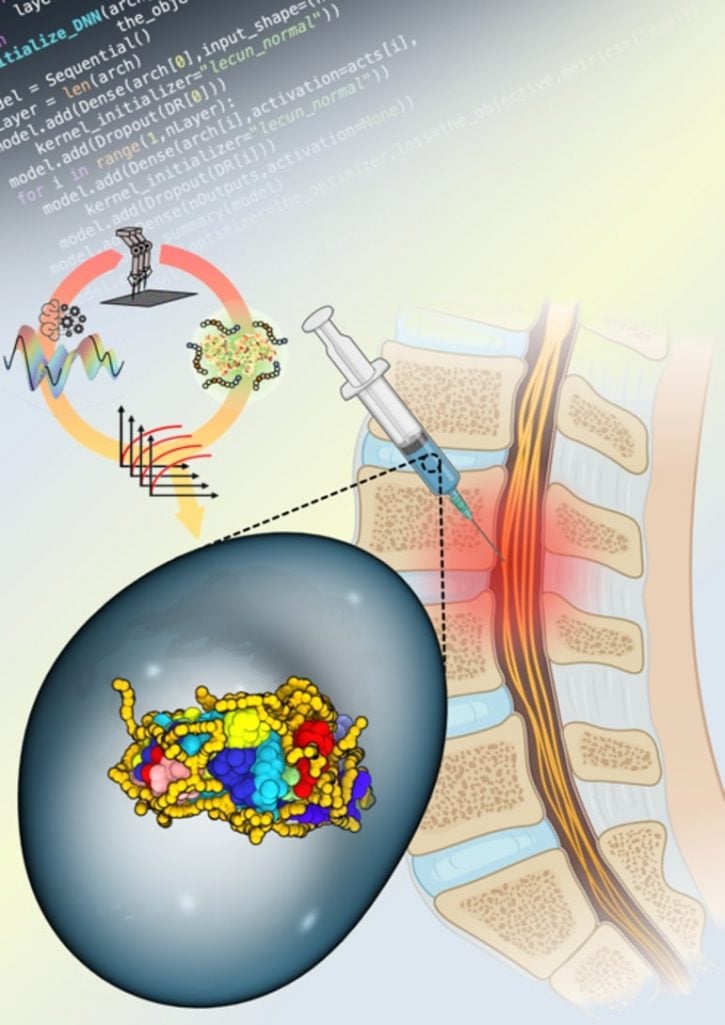
Synthetic copolymers are able to wrap around enzymes such as ChABC and stabilize them in hostile microenvironments. In order to stabilize the enzyme, the researchers utilized an AI-driven approach with liquid handling robotics to synthesize and test the ability of numerous copolymers to stabilize ChABC and maintain its activity at 98.6° F.
While the researchers were able to identify several copolymers that performed well, Kosuri reported that one copolymer combination even continued to retain 30% of the enzyme for up to one week, a promising result for patients seeking care for spinal cord injuries.
Funding: The study received support from grants funded by the National Institutes of Health, the National Science Foundation, and The New Jersey Commission on Spinal Cord research. In addition to Gormley and Kosuri, the Rutgers research team also included SOE Professor Li Cai and Distinguished Professor Martin Yarmush, as well as several SOE-affiliated students. Faculty and students from Princeton University’s Department of Chemical and Biological Engineering also collaborated on the project.
About this AI, Robotics and SCI research news
Author: Emily Everson Layden
Source: Rutgers
Contact: Emily Everson Layden – Rutgers
Image: The image is credited to Rutgers
Original Research: Closed access.
“Machine-Assisted Discovery of Chondroitinase ABC Complexes toward Sustained Neural Regeneration” by Adam Gormley et al. Advanced Healthcare Materials
Abstract
See also

Machine-Assisted Discovery of Chondroitinase ABC Complexes toward Sustained Neural Regeneration
Among the many molecules that contribute to glial scarring, chondroitin sulfate proteoglycans (CSPGs) are known to be potent inhibitors of neuronal regeneration. Chondroitinase ABC (ChABC), a bacterial lyase, degrades the glycosaminoglycan (GAG) side chains of CSPGs and promotes tissue regeneration.
However, ChABC is thermally unstable and loses all activity within a few hours at 37 °C under dilute conditions. To overcome this limitation, the discovery of a diverse set of tailor-made random copolymers that complex and stabilize ChABC at physiological temperature is reported.
The copolymer designs, which are based on chain length and composition of the copolymers, are identified using an active machine learning paradigm, which involves iterative copolymer synthesis, testing for ChABC thermostability upon copolymer complexation, Gaussian process regression modeling, and Bayesian optimization.
Copolymers are synthesized by automated PET-RAFT and thermostability of ChABC is assessed by retained enzyme activity (REA) after 24 h at 37 °C. Significant improvements in REA in three iterations of active learning are demonstrated while identifying exceptionally high-performing copolymers.
Most remarkably, one designed copolymer promotes residual ChABC activity near 30%, even after one week and notably outperforms other common stabilization methods for ChABC.
Together, these results highlight a promising pathway toward sustained tissue regeneration.
Credit: Source link



