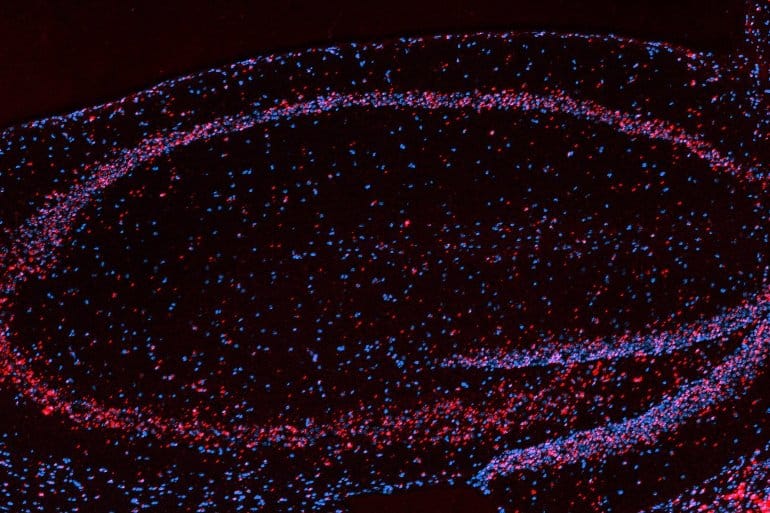
Summary: Floods of calcium that originate from hippocampal neurons can also boost learning, a new study reports.
Source: Columbia University
Scientists have long known that learning requires the flow of calcium into and out of brain cells. But researchers at Columbia’s Zuckerman Institute have now discovered that floods of calcium originating from within neurons can also boost learning. The finding emerged from studies of how mice remember new places they explore.
Published today in Science, the new research doesn’t suggest that you should drink more calcium-rich milk to pass that math class. It provides a better understanding of the mechanisms that underlie learning and memory: knowledge that could help shed light on disorders such as Alzheimer’s disease.
“The cells we studied in this new work are in the hippocampus, the first area of the brain affected by Alzheimer’s disease,” said Franck Polleux, Ph.D., a principal investigator at Columbia’s Zuckerman Institute. “Understanding the basic principles of what allows these brain cells to encode memory will provide tremendous insights into what goes wrong in this disease.”
The brain’s ability to learn and remember—everything from our first words and steps to where we parked our car or left our keys—depends on the gaps where neurons connect to each other, called synapses. Synapses, through which cells exchange information, can be modified over time. This malleability to experience, known as plasticity, relies on how calcium ions flow within the brain.
Nearly all research into the part that calcium plays in plasticity has focused on how it can rush into and out of a synapse through channels on the surfaces of neurons. For more than two decades, scientists have suspected that stockpiles of calcium within neurons might also play a major role in shaping plasticity. But until now, scientists had no way to investigate the effects that calcium discharged from these internal reservoirs had within the mammalian brain.
“For a long time, there were no good tools out there to really probe this intracellular calcium release in a living animal as it learned,” said postdoctoral researcher and first author Justin O’Hare, Ph.D., in the Polleux lab and the lab of Attila Losonczy, MD, Ph.D., at Columbia’s Zuckerman Institute.
In the new study of mice, the Polleux lab and the Losonczy lab focused on the hippocampus, a seahorse-shaped region of the brain central to memory. Specifically, the scientists analyzed pyramid-shaped neurons that can encode memories of locations, called place cells, in the hippocampal region known as CA1.
“Place cells are one of the key tools with which we not only create maps of the world but also associate a place with something, such as a reward, a color, a smell, anything,” said Dr. Polleux, who is also a professor of neuroscience at Columbia’s Vagelos College of Physicians and Surgeons. “The big question is, ‘How are these cells doing this?’”
To answer this question, the researchers had mice run on treadmills with belts made of three different kinds of fabric and decorated with sequins, furry pompoms and other ornaments. These decorations provided visual and tactile sensory cues about specific places on the belts. Place cells in the brains of those mice had been genetically modified to switch on in response to laser light, a technique known as optogenetics. This allowed the researchers to tune those place cells to specific spots on the belts.
Inside place cells, the researchers focused on a gene called Pdzd8. It encodes a protein that normally helps limit the amount of calcium released from the endoplasmic reticulum (ER), an elaborate network of tubes within the cells.
“The ER stores a huge amount of calcium,” Dr. Polleux said. “It’s like a calcium bomb inside all cells.”
The researchers deleted Pdzd8. This deletion removed the brakes on calcium release from the ER. The scientists next looked for changes in the activity of the place cells in both the cells’ central bodies and their dendrites, the treelike branches with which cells receive signals from other cells.
“Any one of the technologies we used to perform these experiments is difficult on its own. Combining them is just nuts,” Dr. Polleux said. “This is probably one of the most challenging sets of experiments that has come out of my lab, and it would have never happened without a deep collaboration with the Losonczy lab and the incredible experimental and analytical talents of Dr. Justin O’Hare.”
The scientists found that increasing the amount of calcium released within a place cell significantly widened the area to which it was attuned, increasing the size of the location it helped a mouse remember. Boosting intracellular calcium release also dramatically increased the duration that a place cell was attuned to a specific location.
“Intracellular calcium release can act like a turbocharger for plasticity,” Dr. Polleux said. “We found that it also makes place cells perhaps even too stable if left uncontrolled.”
The scientists also found the dendrites at the apex of each pyramid-shaped neuron in CA1 are normally all tuned to different places. Increasing the amount of calcium released within these neurons helped attune many of the dendrites at their apexes to a single place during learning but had less of an effect on dendrites at the base of the neurons. Discovering the ways in which all the components of these extraordinarily complex neurons change during learning could help researchers decipher how these cells work.
“Dendrites have long been suspected to function as ‘cells-within-cells’ that can work independently or, when needed, together to enhance the computational power of single neurons,” Dr. Losonczy said. “Our study not only shows that this is indeed the case, but it also provides a molecular mechanism for how this dendritic cooperation is regulated in the behaving brain.”
“Each potential place cell probably receives tens of thousands of inputs carrying information about a space,” Dr. O’Hare said. “If you think about all this complexity, you can appreciate that even a single neuron in the brain is basically like a supercomputer.”
See also
Future research can explore what effects deleting Pdzd8 has on behavior in general. “Recently a paper came out that for the first time identified mutations in Pdzd8 in humans,” Dr. Polleux said. “The individuals that carry those mutations have severe learning and memory deficits, showing how important it is for the brain.”
Dr. O’Hare and his colleagues are now investigating what happens to CA1 in a mouse model of Alzheimer’s disease.
“What’s happening to place cells as this disease progresses? It’s still not known,” Dr. O’Hare said. “Understanding the basic principles endowing place cells with the ability to encode memories in the hippocampus could have enormous consequences for our understanding of what goes wrong in this disease. Then we can think about how that might translate into new therapies.”
About this memory and learning research news
Author: Press Office
Source: Columbia University
Contact: Press Office – Columbia University
Image: The image is credited to Stephanie Herrlinger/Columbia’s Zuckerman Institute
Original Research: Closed access.
“Compartment-specific tuning of dendritic feature selectivity by intracellular Ca2+ release” by Justin O’Hare et al. Science
Abstract
Compartment-specific tuning of dendritic feature selectivity by intracellular Ca2+ release
Dendritic calcium signaling is central to neural plasticity mechanisms that allow animals to adapt to the environment. Intracellular calcium release (ICR) from the endoplasmic reticulum has long been thought to shape these mechanisms. However, ICR has not been investigated in mammalian neurons in vivo.
We combined electroporation of single CA1 pyramidal neurons, simultaneous imaging of dendritic and somatic activity during spatial navigation, optogenetic place field induction, and acute genetic augmentation of ICR cytosolic impact to reveal that ICR supports the establishment of dendritic feature selectivity and shapes integrative properties determining output-level receptive fields. This role for ICR was more prominent in apical than in basal dendrites.
Thus, ICR cooperates with circuit-level architecture in vivo to promote the emergence of behaviorally relevant plasticity in a compartment-specific manner.
Credit: Source link